In Part 1 of this blog series, Elisavet [EDIT Lab first-year PhD student] and Elena [EDIT Lab 2020-2021 MSc student] outlined the inherent limitations of not including more diverse – and thus representative – samples in GWASs. In Part 2 below, they discuss the ongoing efforts towards diversifying research at three different levels.

Elisavet ( EDIT Lab 1st year PhD student)

Elena (EDIT Lab 2020-2021 MSc Student)
NB: This blog discusses concepts like ancestry, race, and ethnicity. This EDIT Lab blog by Dr Yasmin Ahmadzadeh is a great reference for the distinction between these terms.
Improving Diversity in Genetics research: What is being done now?
At the moment, genome-wide association studies (GWASs) can tell us about genetic variants associated with phenotypes in populations of specific descent (i.e., primarily European), with limited generalisability to other populations. Using findings from European GWAS to inform prevention and treatment interventions is likely to improve medical approaches in populations of European descent (1). However, attempting to apply such findings to the medical treatment of people of non-European descent may be both inaccurate and ineffective (2), thus serving to widen health inequalities across the globe. Notably, the understudied populations in GWAS are also the ones with the highest prevalence of mental health problems in countries leading in genetics research (3,4).
There have been many efforts to diversify GWASs, which has resulted in an increase in the number of studies of and participants from non-European ancestral groups, especially over the past 5 years (see charts below). However, the number of studies in European ancestral groups has increased simultaneously, keeping the proportions of studies and participants largely unchanged. Therefore, levelling the existing inequalities by diversifying research (in well-powered studies) is an important issue that can be addressed by researchers, funders, and the public.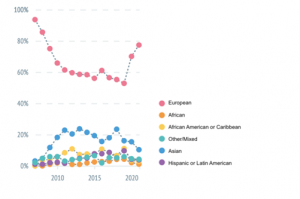 Figure 1. The yearly percentages of genome-wide association studies by ancestral population since 2007, as of the 27th of October and as reported by the real-time Diversity Monitor (5).
Figure 1. The yearly percentages of genome-wide association studies by ancestral population since 2007, as of the 27th of October and as reported by the real-time Diversity Monitor (5).
Researchers.
Researchers are in a crucial position. They could begin diversifying their research initiatives by working with understudied and underrepresented populations. As mentioned earlier, GWAS benefit most from large sample sizes. However, the few studies conducted in understudied populations, although relatively small, have contributed to levelling the existing ancestral imbalances in GWAS findings (1). At present, some initiatives are underway to increase the diversity in genetics research, such as:
The Genes & Health Study: Aims to investigate the links between genes and health records in people from Bangladeshi and Pakistani origins living in East London and Bradford. Fun fact: King’s College London is proud to be involved in this initiative and to have generated GWAS data from more than 50,000 DNA samples in our Genomics and Biomarker core facility laboratory at the Social, Genetic and Developmental Psychiatry Centre (pictured on the right)!
 The Office of Training, Diversity, and Health Equity (TiDHE): A recently established office at the National Human Genome Research Institute that aims to foster diversity and health equity in the workforce of genomics and genomic medicine.
The Office of Training, Diversity, and Health Equity (TiDHE): A recently established office at the National Human Genome Research Institute that aims to foster diversity and health equity in the workforce of genomics and genomic medicine.
Born in Bradford – We are Family: Aims to collect genetic and phenotypic data to investigate patterns of health and illness in over 30,000 people born in Bradford, one of the UK’s most diverse cities.
Human Heredity & Health in Africa (H3Africa): Aims to facilitate a contemporary research approach to the study of genomics and environmental determinants of common diseases with the goal of improving the health of African populations. As human species originated in Africa and migrated to the rest of the world, studying African genomes can inform our understanding of our origins, migratory patterns, and the genetic diversity that exists today.
NeuroGAP-Psychosis: Aims to conduct GWAS on individuals from Ethiopia, Kenya, South Africa and Uganda with schizophrenia or psychosis (6).
GenomeAsia 100K Project: Aims to sequence and analyse 100,000 Asian individual genomes to accelerate Asian population-specific medical advances.
100,000 Genome projects: Following the completion of the 100,000 Genome Project in the UK in 2018, nine other countries from around the world have joined this effort to sequence more than 100,000 genomes each. These countries include China, Saudi Arabia, Dubai, Australia, Estonia, Japan, USA, France, and Turkey (7).
In addition, researchers can also ensure the public dissemination of their GWAS findings by making them available to the research community and the public.
Funders.
Whilst researchers detect and investigate gaps, only funders can finance the necessary work. As such, funders could prioritise studies focused on understudied ancestral groups. Already, large funding bodies such as the National Institute of Health in the USA and the Wellcome Trust in the UK have funded projects such as H3Africa (mentioned earlier), and are helping lead the efforts underway.
Everyone.
All individuals should be mindful of the tools, terminology, and language used when measuring diversity and when discussing results from genetics research. It is important to distinguish between race, ethnicity, and ancestry when discussing research findings, as these may lead to unintentionally racist narratives.
Most of all, making the field of genetics research more diverse requires collective effort, from individuals within and beyond academia. Similarly to researchers, all of us could familiarise ourselves with the appropriate terminology relating to race, ethnicity, and ancestry in order to ensure their correct interpretation and critical appraisal of available information.
It is worth highlighting the power that the public holds. Each one of us could play a unique, yet essential, role in this effort. As either participants, advisory board members (e.g., READ group), or advocates, members of the public can support ancestrally diverse research initiatives that are crucial in making genetics research more representative. Even as a reader, beginning to understand the complexities of genetics research and ancestrally diverse populations is a step in the right direction.
This month, and every month, we hope to continue to make our research efforts more inclusive, diversify mental health initiatives, and ultimately transform treatment, one GWAS at a time.
References
- Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019 Apr;51(4):584–91.
- Sirugo G, Williams SM, Tishkoff SA. The Missing Diversity in Human Genetic Studies. Cell. 2019 Mar 21;177(1):26–31.
- Mental Health Foundation. Black, Asian and minority ethnic (BAME) communities [Internet]. Mental Health Foundation. 2021. Available from: https://www.mentalhealth.org.uk/a-to-z/b/black-asian-and-minority-ethnic-bame-communities
- National Institute of Mental Health. Mental Illness [Internet]. National Institute of Mental Health. 2020. Available from: https://www.nimh.nih.gov/health/statistics/mental-illness
- Mills MC, Rahal C. The GWAS Diversity Monitor tracks diversity by disease in real time. Nature Genetics. 2020;52:242–3.
- Stevenson A, Akena D, Stroud RE, Atwoli L, Campbell MM, Chibnik LB, et al. Neuropsychiatric Genetics of African Populations-Psychosis (NeuroGAP-Psychosis): a case-control study protocol and GWAS in Ethiopia, Kenya, South Africa and Uganda. BMJ Open. 2019 Feb 1;9(2):e025469.
- Philippidis A. 10 Countries in 100K Genome Club [Internet]. Clinical Omics. 2018. Available from: https://www.clinicalomics.com/topics/translational-research/biomarkers-topic/biobanking/10-countries-in-100k-genome-club/
