Entries are now open for the HSDTC Public Engagement Competitions 2021
HSDTC Science Communication Competition 2021
How well can you communicate your research and its significance to non-experts? Submit your entry in a newspaper style article for the chance to win £300 and be featured on our website and blog.
We’re also running a workshop called Science Communication: Writing for a Lay Audience on Thursday 4 February. Book now for the chance to learn how to summarise your doctoral research and write great articles for a general audience: invaluable for communicating your science to the public and for entering the HSDTC Science Communication Competition!
HSDTC Science Image Competition 2021
An annual contest to find the best images that represent the excellent doctoral research that goes on across the Health Faculties at King’s, with the chance to win £300 and culminating in an exhibition and awards ceremony.
HSDTC Science Communication Competition 2020
Doctoral research students at King’s were invited to submit a short ‘newspaper style’ article on their research topic.
The article must have been based on the research they are currently engaged with, or that their group is doing, whether that be the whole project or one aspect of it.
The article should have been aimed at a non-specialist audience and be understandable to an interested member of the public.
The judges were looking for articles which: are compelling to read and easily understandable; clearly explain the research being done; answer the question “why does this research matter?”; and are worthy of publication in a national newspaper.
The five shortlisted entries, including our winner, Sogol Salamipour, and our runner-up, Rachel Potterton, are published here:
Rachel Potterton (runner-up)
Institute of Psychiatry, Psychology & Neuroscience
“I’m not a teenager, I’m 22. Why can’t I snap out of it?” Seeking Help for an Eating Disorder During Emerging Adulthood
Eating disorders (EDs) are serious mental illnesses characterised by disturbances of body image and eating behaviour, experienced by approximately 8% of women and 2% of men during their lifetime.
For most illnesses, professional help is sought shortly after symptoms appear. But those with EDs tend not to seek help quickly enough; Beat, the UK’s largest ED charity, finds the average waiting time to be 2.9 years. During these years of suffering alone, ED sufferers are at particular risk of depression, suicide and a range of physical health complications.
At the Section of Eating Disorders, KCL, our study began with the observation that ‘emerging adults’ (18 to 25-year-olds) are particularly likely to delay help-seeking. We interviewed 14 emerging adults with EDs, finding a consistent set of themes and experiences among them.
Many participants falsely believed that EDs are only possible for those with extreme low-weight and only affect “white teenage girls”. These beliefs contributed to reluctance to seek help amongst those who don’t fit that description. As one participant reported, “I thought if I told any of my friends, they’d just laugh in my face”.
Furthermore, we found that emerging adults often thought they were “too old” to have an ED: “I’m not 16, I’m 22. It’s a stupid issue, and I’m ashamed of it.” Instead of seeking professional help, emerging adults tried to “fix” their difficulties themselves, frequently seeking resources online.
This research furthers our understanding of delayed help-seeking amongst emerging adults, highlighting that overly simplistic beliefs about EDs contribute to reluctance to seek help in this population.
Whilst qualitative research such as this gives us a detailed picture of the experiences of a group of people, quantitative research is needed to explore if these findings can be generalised beyond the study’s sample.
Should these results be replicated, policy aimed at connecting patients with professionals faster might usefully focus on diffusing false assumptions around the ethnicity, gender and age of those at risk of an eating disorder.
David Rosen (shortlisted)
Faculty of Life Sciences & Medicine
New Research Reveals Weaknesses In Cardiovascular Health Check Screening
The Department of Health’s cardiovascular screening program has had a chequered history. Despite substantial long-term investment, it has consistently failed to reach its outcome improvement targets and is seen by many in the Department of Health as not fully justifying its ongoing financial commitment. Meanwhile evidence regarding its failings have continued to mount. A recent paper from King’s College London has revealed new evidence explaining why some patients appear to remain unconvinced when given their test results, with many at high-risk failing to adopt potentially life-saving recommendations.
David Rosen the research paper’s author believes that when risk information is communicated to patients, health professionals assume that individuals will act rationally and in their own best interest. The results from this latest research reveals that patients in fact rarely act rationally when given bad news about their health. In his best-selling book Thinking Fast, Thinking Slow, Nobel Prize winner Daniel Kahneman describes how individuals often fall prey to a raft of cognitive biases when under stress. These can subvert decision making and lead individuals to make flawed and potentially dangerous choices.
Rosen’s research shows that many of these biases appear to be in play during the Health Check process, particularly when the test results are considered ambiguous, confusing or lacking personal relevance. However, when patients were asked to consider the consequences of their inaction and encouraged to consider how much they might regret placing themselves and their family at risk, they were over 40 times more likely to show positive intentions to adopt a more healthy lifestyle. The inclusion of emotive drivers such as anticipated regret can elicit powerful emotive imagery and may be a useful future tool within risk communication.
This latest research reveals powerful new evidence describing how patients respond to risk information regarding their heart health. It comes at a crucial time, when ministers are under pressure to improve the UK’s performance in managing chronic disease and reinforcing the UK’s role as a centre of excellence. This report is likely to be read with interest in the coming days.
Sogol Salamipour (winner)
Faculty of Life Sciences & Medicine
Can we predict women who will deliver prematurely?
Premature birth is the biggest cause of death in children under the age of five, and each year, it is estimated that 15 million babies are born too soon. Despite extensive research and new treatment options, in almost all countries that have data available, this number is still rising. Whilst many of these babies survive, they are at an increased risk of devastating long-term health conditions.
The good news is that there is treatment available. Three quarters of premature birth deaths could be prevented with our current interventions. So, what exactly is the problem?
The problem is in fact identifying the women who are at risk of delivering early and pinpointing who it will happen to so that they can receive the right treatment.
Researchers at King’s College London have been trying to find ways to predict premature birth. A new technique that recreates a 3D model of the vaginal environment in the laboratory has been developed and could help scientists understand what exactly happens at the cellular level that may be contributing to early birth.
Recent research has shown that the bacteria in the vaginal environment are different in women who deliver prematurely. This finding could be important as different bacteria will generate and leave behind a different metabolic pattern. The group at King’s College London has already started to use their novel laboratory model to test this and have found that certain bacterial metabolites affect the vaginal cells.
So, could the imprint that our microbe friends leave behind, just like fingerprints, help scientists identify who will deliver prematurely? One thing is for sure, although it is still in early stages, the development of such models within the laboratory gives scientists more tools to address this global problem and get a step closer towards finding better clinical tests to predict women who will deliver prematurely and prevent neonatal loss and the devastation that comes with it.
Chantelle Spiteri (shortlisted)
Faculty of Dentistry, Oral & Craniofacial Sciences
Organoids: Organs in a dish. It’s Alive!
Frankenstein. To many people, uttering the name of the scientist who built a monster by mix and matching organs together sends a shiver down their spine. To others, it makes them think. What if we had an unlimited supply of organs to experiment with!?
Scientists have come up with such a solution in the form of organoids, which are small clusters of cells functioning a bit like organs. The amazing thing about organoids is that they provide a simple way to model how human organs function, making them a powerful tool to understand diseases such as cancer and Alzheimer’s. Despite the progress, researchers are struggling to control how cells arrange themselves within the organoid other than in radial symmetry. That is the cells on the outside are the same as each other but different from those on the inside. Radial symmetry can be better explained by thinking of an orange cut in half. It consists of the peel surrounding a white fluffy layer which is protecting the fruit. The circular arrangement of these layers represents radial symmetry. The problem is, that without controlling the cells’ arrangement we remain unable to develop fully functional organoids.
So what can be done to develop more representative organoids? Our group at King’s College London has proposed an innovative way to break symmetry. We are growing cluster of cells surrounded by nanoparticles and a few chemicals that need to be delivered inside the cells. Next, we shine a laser at individual cells within the cluster to activate a handful of the surrounding nanoparticles. Fueled with the laser energy, the activated nanoparticles can rip holes in the nearby cells, allowing for those outside chemicals to make their way through. Once inside, a sequence of events is initialized causing functional changes of that cell and those around it, starting from a single selected point.
In a nutshell, the goal is to organize cells within organoids and build the organ-like structures for the labs of tomorrow. Who knows, maybe patching up a Frankenstein’s monster will not be a myth after all.
Sergio Villicaña Muñoz (shortlisted)
Faculty of Life Sciences & Medicine
DNA Methylation: the conductor of the symphony of life
Have you ever listened to Beethoven’s Ninth Symphony? A marvellous interweaving of instruments and voices, the striking melodies and vibrant rhythms were all encoded by Beethoven in a very complex score. However, none of us would know how it sounds without the guidance of a conductor. Conductors are primarily responsible for unifying the performance, setting the tempo, listening critically and ensuring synergy across musicians.
We, as individuals, are somewhat similar to the Ninth Symphony: our genome is the score while a biological process, namely DNA methylation, directs the music. DNA methylation is a chemical modification of our DNA that plays a role as a ‘regulator’ of genes, increasing or decreasing their action and expression into proteins—just as the conductor ‘interprets’ a score and brings music to life.
Recently, scientists at King’s College London studied how DNA methylation patterns are established, and showed that changes in our genome can influence DNA methylation. These changes, or single nucleotide polymorphisms (SNPs), are variants of ‘notes’ in our DNA code. SNPs make us unique among billions of humans, but can also cause diseases, although it is not always clear how—while some SNPs can disrupt genes directly, many others have less clear consequences. The discovery that SNPs can alter DNA methylation is important as this identifies biological effects on gene regulation, which can also contribute to disease.
In twins, DNA methylation studies are particularly intriguing, because genetically identical twins share their genome, but the methylation patterns of their DNA differ. In fact, to find the genetic drivers of methylation, researchers at King’s work with data from TwinsUK, the largest twin registry in the UK. With them, they test for links between hundreds of thousands of methylation signals against millions of SNPs.
Like a musician performs better at a concert if he knows the score, identifying the interplay between SNPs and DNA methylation can help towards understanding the biological pathways driving human disease susceptibility, and thus, improve their prevention, early diagnosis, and treatment.
And so the music of life can go on!
HSDTC Science Image Competition 2020
Doctoral research students at King’s were invited to submit an image which tells their research story.
Submitted images needed to convey either the totality, or an aspect, of the research that they do. Images could have shown, for example, the subject matter of their research, such as an image of cells or the brain, or research in action, for example the methods, equipment or facilities used in their research.
We were looking for images which might explore the social impact that their research has on a global, population, or individual scale. They may be a true representation of the subject, or manipulated post-production, such as with false colouring; or they could show their research in a more abstract way.
We were open to entries created by many techniques, including but not limited to: photography; microscopy; medical imaging; new and emerging imaging techniques; data visualisation; artistic media.
The judges were looking for images which are informative, captivating, and which clearly communicate the research being done.
The six shortlisted entries, including our winner, Kathryn Dalrymple, and our runner-up, Nicholas Groth Merrild, are published here:
Ieva Berzanskyte (shortlisted)
Institute of Psychiatry, Psychology & Neuroscience
Ability to hack cell connectivity is the key to repairing broken neural networks in human disease and trauma.
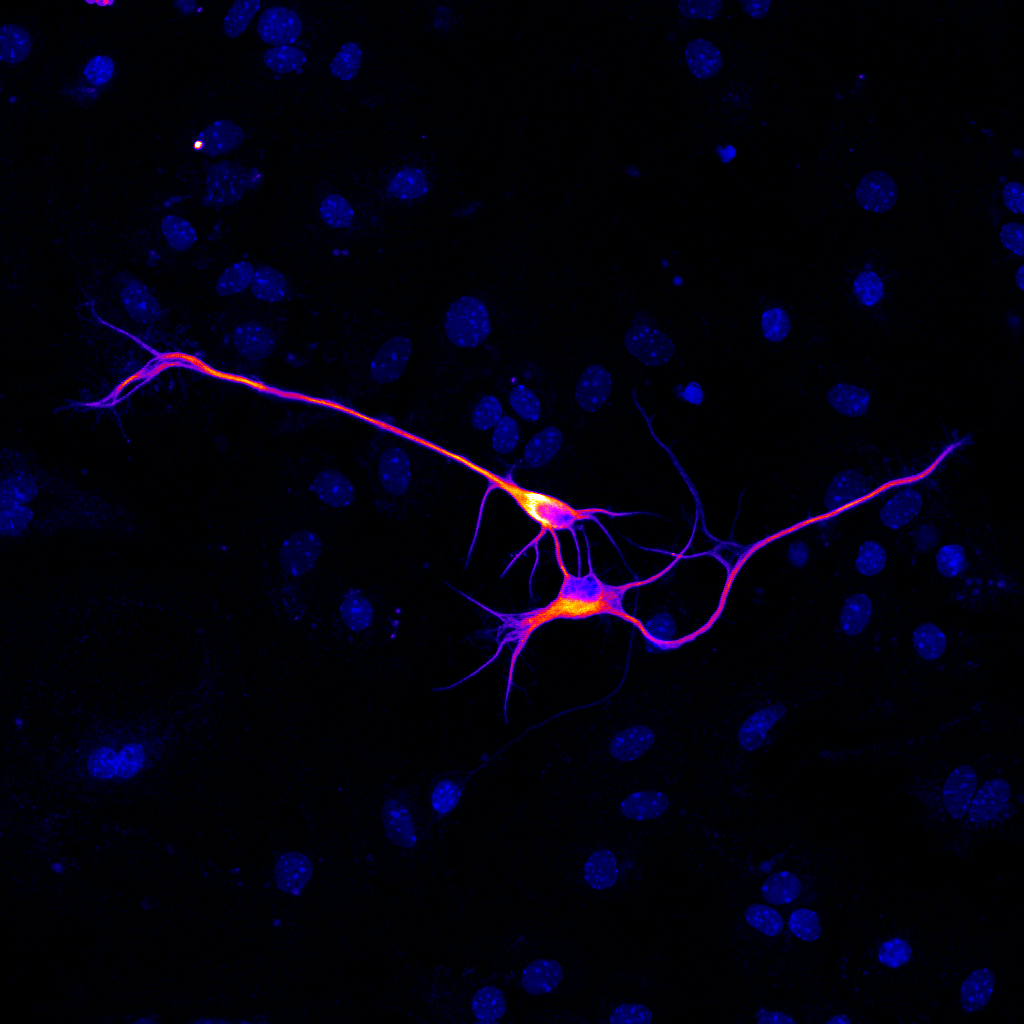
Human motor neurons derived in the lab from human embryonic stem cells were plated on a plastic dish coated with protein-rich substrate and neuron growth supportive cells – astrocytes (blue nuclei around the two cells).
Neurons were tagged with an immunofluorescent label for a protein expressed specifically in these motor neurons – choline acetyltransferase. Image was acquired using a confocal fluorescence microscope using ~400 times magnification, and processed with an imaging software Fiji to represent a heat map of protein label intensity in different areas of the cell.
Kathryn Dalrymple (winner)
Faculty of Life Sciences & Medicine
The shared family environment: determinants of food habits in early life.

This photo was taken in Nepal last summer, of a brother and sister sharing a snack whilst they waited for their mother who was in a local shop. Our dietary habits are influenced by a number of factors, including but not limited to genetics, social and environmental determinants.
The importance for public health and our dietary behaviours is that once these behaviours are developed in early life they track across the life crossed into adulthood. Therefore, if we develop unhealthy habits as a child these tend to stay with us for the rest of our lives.
Recent work published by the Department of Women and Children’s Health at Kings College London highlights that already by age 3, ‘unhealthy habits are associations with higher rates of obesity in children, this includes being more responsive to food, lower feelings of fullness and a high intake of processed and snacking foods.
This photo highlights the role of a shared family environment and food intake and how from a young age we are influenced by a variety of exposures which in turn affect our food intake. This photo was taken on an iPhone, with no post-production editing.
Andrew Doel (shortlisted)
Faculty of Life Sciences & Medicine
Lactation Counselling, The Gambia

The WHO recommends exclusive breastfeeding of infants for the first 6 months of life and continued breastfeeding up to two years or beyond, owing to its well established benefits for both mother and infant.
This picture shows a National Nutrition Agency (NaNA) lactation Counsellor, delivering UNICEF accredited infant and young child feeding (IYCF) counselling to a group of new mothers in The Gambia, West Africa.
Levels of breastfeeding in The Gambia are generally good, to a large extent due to it being a cost free form of nutrition for the infant, in a low income context. In recent years, however, urban regions of the country (such as where this picture was taken) have exhibited the beginnings of a ‘Nutrition Transition’, characterised by a move away from traditional diets in preference of westernised foods and altered dietary behaviours. One aspect of this transition is an increase in the use of formula milk and a shift away from WHO recommended IYCG practice. This shift highlights the importance of understanding as clearly as possible, the benefits of breastfeeding in order to support organisations such as NaNA in their efforts to support its promotion.
To this end, a study being conducted by King’s college staff based at the Medical Research Council Unit, The Gambia (MRCG) aims to establish Reference Values (RVs) for micronutrients in human milk. The RVs created will be used as an international reference against which to compare concentrations in different population groups, and it is anticipated that they will become an international standard to be used, for example, to evaluate the effects of nutritional interventions, such as maternal supplementation or national fortification programmes, such as those being conducted by NaNA.
The image was taken on a canon DSLR camera with no post-production manipulation.
Nick Gatford (shortlised)
Institute of Psychiatry, Psychology & Neuroscience
Actin Filaments in the Growth Cone of a Young Human Neuron

The growth cone is an intricate and complex structure that helps neurons grow and connect during brain development. The internal skeleton of this structure consists of thread-like filaments made of a protein called actin. The latest advancements in human stem cell biology and super-resolution microscopy have allowed us to see these filament structures in developing human neurons in unprecedented detail.
This image shows actin filaments in the growth cone of a young human neuron imaged using structured illumination microscopy at the Wohl Cellular Imaging Centre. The image was deconvolved in Nikon Elements to improve image clarity. Brightness/contrast were then adjusted in ImageJ and a filter was applied to highlight signal intensity. Images such as this offer new understanding of both healthy growth cones and dysfunctional growth cones in brain disorders such as autism spectrum disorder.
Geraldine Jowett (shortlised)
Faculty of Oral, Dentistry & Craniofacial Sciences
Two Faced
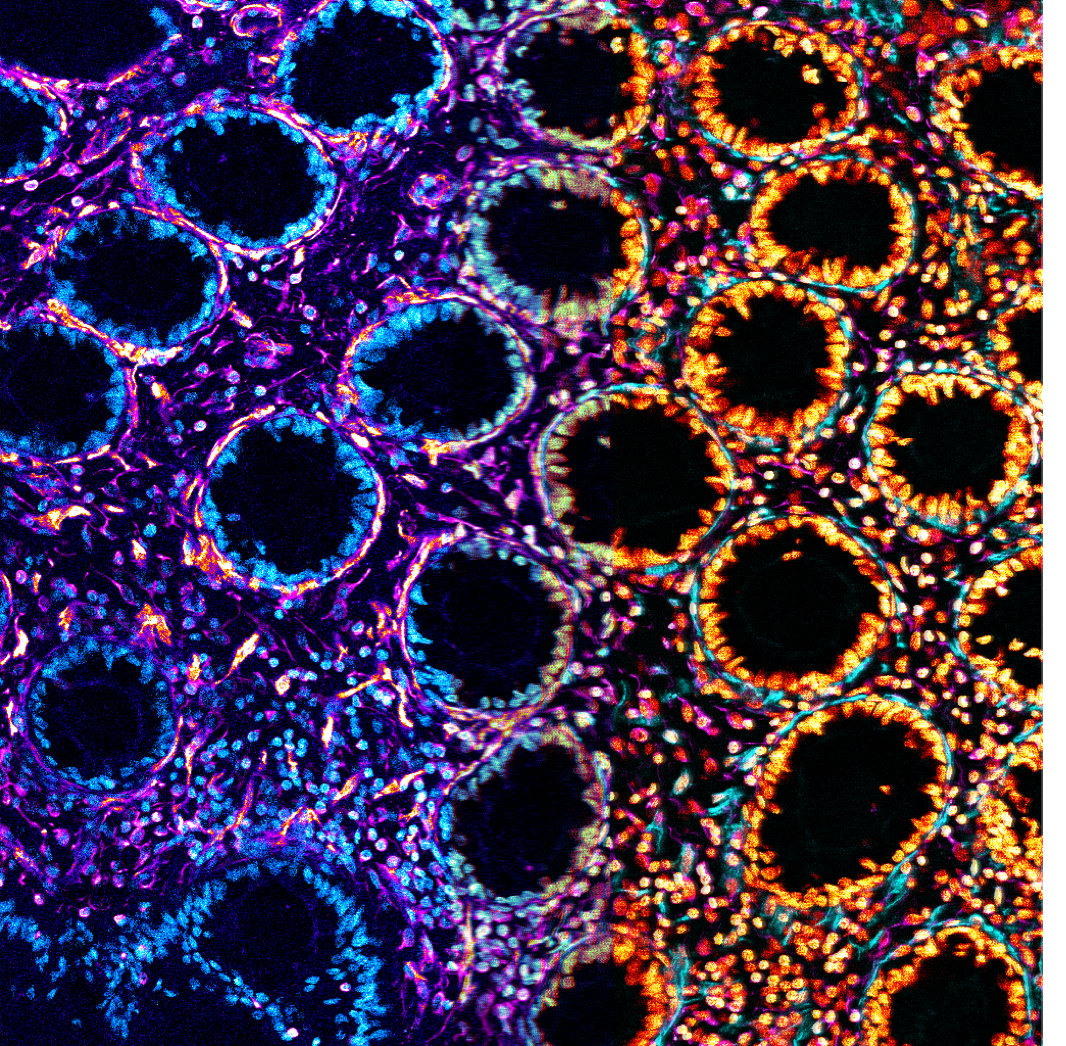
Inflammatory type-1 innate lymphoid are a very rare, tissue resident cell type that accumulate in inflamed patient tissues, but we don’t know why they are enriched or what they do. It was assumed they drive primarily inflammation, as they secrete cytotoxic Interferon-, but in my PhD I have discovered that they also secrete anti-inflammatory TGFβ, and that their main impact on the intestine outside the context of acute infection is driving growth and matrix deposition by supporting cells, not cell death.
This is a confocal image of a 20X magnified cryo-section of colon from a patient with Crohn’s Disease. It shows the rings of epithelial crypts, but focuses on the smooth muscle and matrix depositing fibroblasts around them. The image was split in two and overlayed after two different false colourings in FIJI of the immunocytochemically revealed SMA (Magenta left, Cyan right), Vimentin (Orange left, Magenta right), and Nuclei (Cyan dapi left, Orange right), as an artistic representation of the soothing and inflammation.
Nicholas Groth Merrild (runner-up)
Faculty of Oral, Dentistry & Craniofacial Sciences
Hanging by a Thread
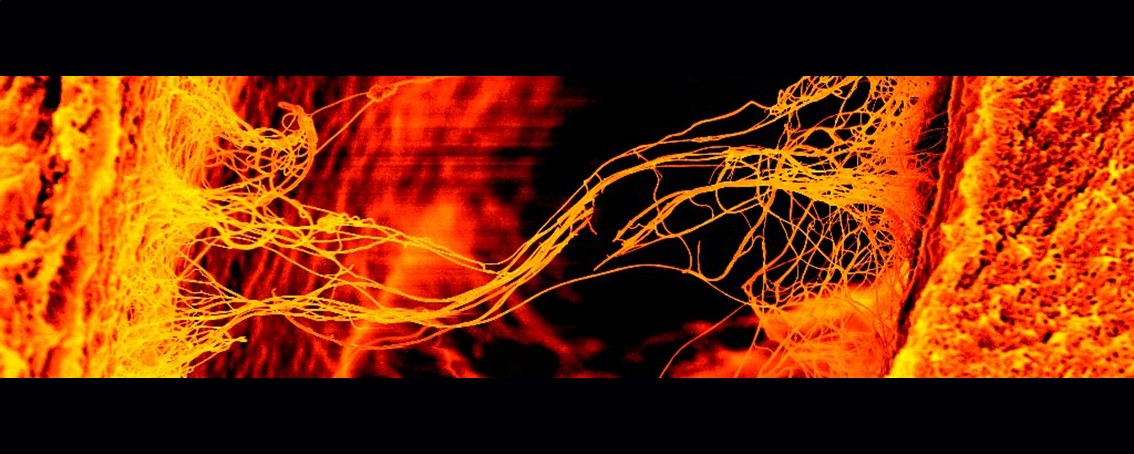
Comprised of a web of highly dense, organised and beautiful rope like structural proteins, collagen carries and distributes the tension of the loadbearing connective tissue of joints – articular cartilage (AC). If adult AC is fractured, or otherwise lacerated, reintegration of the two parts is highly unlikely. With no integration, load distribution is suboptimal and contributes to the tissue’s further decline, a condition known as osteoarthritis. This lack of ability to integrate remains a problem for implanting donor cartilage into a host cartilage lesion, as the two will not integrate. It may therefore be that collagen, the glue that holds the tissue together, may be the key to successful integration and tissue longevity. It is quite fitting that the Greek root for collagen is Κόλλα (kolla), literally – glue.
Using a juvenile pig AC wound model in vitro, we can successfully observe integration of the two halves. Having ruled out the generation of new large structural proteins (e.g. no new collagen), no new enzymatic cross-linkers associated with collagen linking, or any macro molecules generally associated with aiding cross-linking, the project appeared to be hanging, literally, by a thread. We have, however, recently identified a promising protein we believe to be contributing to successful integration and remodelling of the existing collagen network. Upon confirming its importance, we aim to leverage this protein for tissue engineering strategies.
Using scanning electron microscopy, this image (width just under 50μm; ~1/20th of a millimetre) shows individual collagen fibres averaging ~100nm (~1/10,000th of a millimetre!) spanning from one cartilage surface to another. The image was cropped to produce the aspect ratio. The original black/white image was false coloured in the yellow and red colour channels in Fiji, and brightness and contrast were adjusted to produce the final image submitted.








